The parents of children in Turkey with a rare genetic disease have been turning to crowdfunding to meet the costs of a revolutionary new treatment. Spinal muscular atrophy (SMA), a disease affecting the central nervous system that can be fatal if left untreated, has until now been treated in Turkey with Spinraza, a form of medication that the patient must take throughout their life. A new gene therapy treatment, Zolgensma – which only needs to be taken once – was approved in 2019, but it is not yet available in Turkey.
To make matters more complicated, the medication must be taken during a child’s early years, meaning that families in Turkey must raise large sums of money at short notice, to cover the cost of treatment abroad. Without state support – Turkey’s social security system does not currently cover Zolgensma – costs can run above $2 million.
Murat, a one year-old boy who suffers from SMA, has become the face of a crowdfunding campaign launched on Twitter nine months ago. His uncle, Murat Arksak, told Inside Turkey that only around 45 per cent of the money needed for treatment has been raised so far, and that young Murat is already experiencing muscle wasting – a common symptom of the disease.

“He can’t crawl or walk even though he’s a year old. He’s receiving physical therapy at the moment, and is on a steady oxygen supply through a BiPap machine [a ventilator],” Arksak said.
“He’s going to need a tube in his trachea very soon. Unfortunately, he’ll die if he can’t receive the treatment on time.” Children who suffer from Type 1 SMA, like Murat, often die before the age of two if not treated.
Arksak said that the Turkish government should be making the treatment available as part of the domestic health service, but that it has not responded to public petitions on the matter.
“The government unfortunately refuses to take any steps about the issue. There are nearly 200 babies in Turkey that qualify for the Zolgensma treatment and could be cured thanks to it. The state puts sole responsibility on the parents,” he said.
Instead, families try to raise funds through public campaigns, if their regional governor’s office gives permission. “We work with a group of 200 volunteers to save Baby Murat,” said Arksak. “A total of 55 children in Turkey have been able to get the Zolgensma treatment so far and survived.”
Mirza, another one year-old who suffers from SMA, is being treated with Spinraza, the standard in Turkey. He needs a ventilator to breathe, and his treatment is carried out at home, primarily by his mother, supported by physiotherapists and breathing coaches.
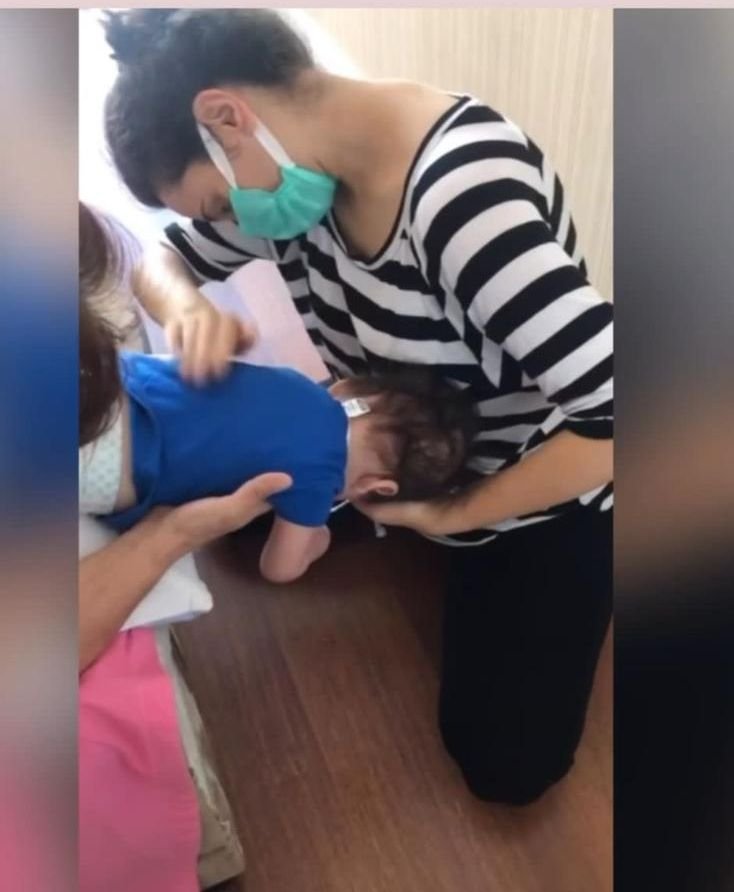
Mirza’s family had to move from the Black Sea district of Ordu to Ankara, Turkey’s capital city, to gain access to treatment, said his Mirza’s father, Barış Kılıç.
“We had to get a place in Ankara. My wife, the kids and my mother stay there. I stayed behind in Ordu to carry on fundraising efforts. We concentrated all our efforts on Mirza’s campaign,” Kılıç said.
Mirza is unable to perform any body movements a child his age is supposed to, his father added.
“He’s always laying down. We try to strengthen his muscles with physiotherapy. He’s not able to sit or hold up his head. Our children need their muscles to be stimulated, so that they don’t deteriorate completely. You’re supposed to rub their muscles and go through their physiotherapy exercises. We have to pat on his back constantly to make sure that saliva doesn’t go into his airways,” Kılıç said.
The drug Spinraza, Mirza’s father said, is only delaying his symptoms, rather than curing him of the disease. “He regains certain movements but he loses them again as soon as the medication wears off. And then there’s the bureaucracy. The government will assign every patient four doses initially, but they need to take an exam to qualify for a fifth dose. They asked him to breathe on his own for four hours, to pull up his knees or hold his head up on his own. Well, he would be a perfectly healthy child if he could do all that!”
Mirza’s family are now planning to take him to the US. “Our fundraising campaign was approved by the governor’s office. The hospital told us it would cost $2,418,000 and so far we have $1.75 million. The genetic treatment was 95 percent effective according to the company’s data, so we trust it as a family. We see babies who receive it, they gain functions that a baby should have,” Kılıç said, adding that he thought the Turkish government should be paying for treatment.
Several children whose families have managed to raise the required funds are showing progress after treatment with Zolgensma.
In July this year, baby Güneş and her family travelled to Boston Children’s Hospital in the US. Her father, Zekeriya Karavil, raised the money in four and a half months, and has been with her for the 14-week treatment.
“We are staying with a couple other families whose kids are receiving the treatment, we got a place in the same building. [There are] families from Giresun, Adana, İzmir, Balıkesir and İstanbul,” he told Inside Turkey. “We surely have been seeing changes in Güneş. She couldn’t open her fingers [before], but three weeks ago, she was able to completely spread her fingers, she could hold out her right arm and lift it.
“Now her hands and arms have been much more active. She’s been turning right and left, she wasn’t able to turn over from one side to the other before. We’ll stay here for another 14 weeks,” he said, adding that their other child is being looked after in Turkey by a grandmother. “I wish we could have received this treatment in Turkey – that way our family wouldn’t have needed to be separated.”
Özge Üstün, a Turkish lawyer who specialises in children’s rights, said that families prefer crowdfunding campaigns over attempting to bring a case in court.
“They go through the fundraising campaigns because they don’t want to deal with the legal process to get the treatment approved in Turkey,” Üstün said, adding that it is a difficult choice nonetheless. “The campaigns are tough, the families have to deal with organizing the fundraising and cope with their child’s health issues at the same time. Sometimes, the children die or experience irreversible muscle loss before the money is collected. Some campaigns move very slowly, and there’s little support, so the families feel alone.”
Üstün suggested that information on the campaigns should be collected centrally – or, even better, the government should begin offering Zolgensma in Turkey itself as part of the welfare state. “The United Nations convention on children’s rights protects a child’s right to access the best healthcare, and this includes developing medical procedures and modern methods of treatment,” she said, adding that the bureaucratic hurdles currently in place for Spinraza should also be removed.
Crowdfunding campaigns have been criticised by the Turkish government. In January 2021 the health minister, Fahrettin Koca, that campaigns to raise money for SMA treatment “made the state seem incapable”. Some families’ social media accounts were then blocked.

According to Utku Çakırözer, a parliamentary deputy for the opposition People’s Republican Party (CHP), the ministry of health is suspicious of gene therapy treatments, even when they have been approved abroad. According to press releases from July and September this year, Çakırözer has been campaigning for Zolgensma to be approved for use in the country.
“Hundreds of children with SMA across Turkey and their families are waiting for their campaigns to be completed to go abroad and start their treatment. Time is running out for them. Turkey should immediately start administering this treatment that’s used in many other countries in the world. This shouldn’t be evaluated from a financial perspective,” he said.

